-
Home > News & Events > Blog > Human Health

Saccharomyces boulardii (S. boulardii) was discovered by French scientist Henri Boulard in 1920. During a cholera outbreak, Henri Boulard noticed that only the people who chewed lychee and mangosteen peels or drank tea made from lychee and mangosteen peels did not develop cholera symptoms. He then isolated a tropical yeast strain named Saccharomyces boulardii from lychee and mangosteen peels. Yeasts are classified based on their physiological and biochemical properties.
S. boulardii was initially identified as Saccharomyces cerevisiae (S. cerevisiae) both on morphology and physiology basis. In 2020, a study found a specific sequence (CAG) 9 that exists only in S. boulardii gene locus 4, and isolated it from other species of S. cerevisiae and identified it as a subspecies. As a subspecies of S. cerevisiae, S. boulardii has similar growth characteristics with S. cerevisiae, but also has some characteristics different from S. cerevisiae.
S. boulardii preparation is a fungal preparation that can be used together with antibiotics and can effectively prevent intestinal diarrhea-related diseases. Its mechanism of action is still under study, but it has been confirmed to have antibacterial, nutritional, immunoregulatory and anti-inflammatory effects. Moreover, it also works by inactivating bacterial toxins, restoring damaged cells and maintaining the integrity of epithelial barrier.
S. boulardii can be used in animal production. As a kind of probiotics, S. boulardii can be used as a feed additive in animal husbandry. In the application of livestock and poultry production, S. boulardii has shown the effects of reducing feed to gain ratio of livestock and poultry, improving production performance, enhancing body immunity, reducing diarrhea, and regulating intestinal flora balance. A large number of studies have confirmed that S. boulardii can increase feed conversion rate, improve meat quality and enhance production performance. Adding 2g/kg of live S. boulardii (2×10^6 CFU/g) to the daily ration of weaned piglets can increase live weight and average daily weight gain of the piglets; adding 2×10^9 CFU/kg of S. boulardii can improve the feed conversion rate of piglets; supplementing piglets with fermented grape pomace containing 30g/kg of S. boulardii (1.0×10^7 CFU/mL) can significantly increase feed intake and improve meat quality of piglets. For calves with diarrhea, adding 1×10^10 CFU/d of S. boulardii can increase the average daily weight gain to the same level as non-diarrheal calves. The average daily weight gain of piglets in the group added 200g/t of S. boulardii was 39.9% higher than that in the control group, and the mortality of piglets induced by Escherichia coli lipopolysaccharide was reduced by 20%. After broiler chickens are infected with Salmonella, supplemental feeding of 1×10^7 CFU/g S. boulardii could inhibit the weight loss and increase the average daily weight gain by 7% compared with the control group. In addition, supplementation with S. boulardii can significantly increase the number of leukocytes, lymphocytes and neutrophils in serum and the concentration of γ-interferon in piglets, decrease the contents of interleukin-1β and interleukin-6, and improve the immunity of piglets.
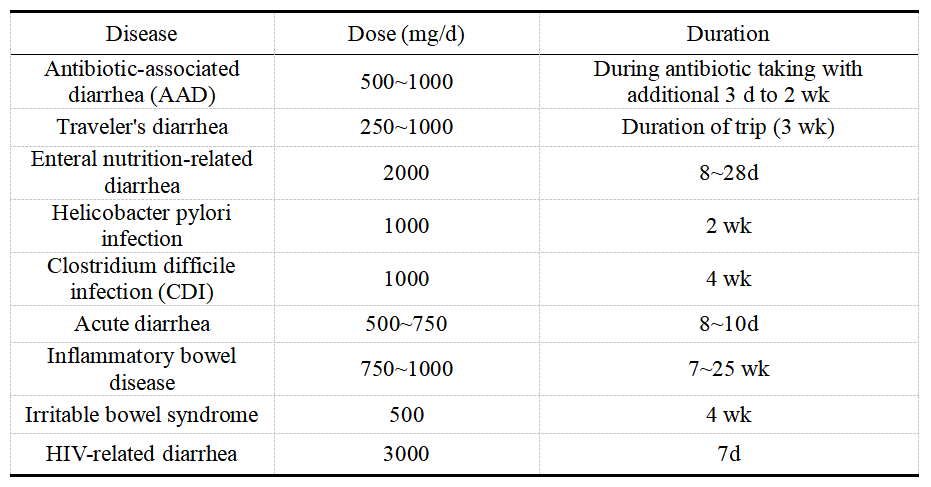
S. boulardii can be used clinically in humans. S. boulardii has been used as a treatment for diarrhea since 1950 and is now commercially available throughout Europe, Africa and South America. S. boulardii has been recommended by the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) and the UK's National Institute for Health and Clinical Excellence (NICE) as well as other institutions for the treatment of various types of diarrhea. Clinical trials have shown that S. boulardii has clinical curative effects on acute diseases such as antibiotic-associated diarrhea (AAD), clostridium difficile infection (CDI), traveler's diarrhea, acute diarrhea, children diarrhea, and enteral nutrition-related diarrhea, and similar clinical efficacy for Crohn's disease, ulcerative colitis, irritable bowel syndrome, and HIV-related diarrhea (Table 1). A double-blind placebo trial study of 269 children aged 6 months to 14 years who received antibiotic treatment found that diarrhea occurred in 23% of those treated with antibiotics and placebo, compared with 8% of those treated with antibiotics or S. boulardii. McFailand et al. comprehensively analyzed 12 studies on the effect of probiotics in preventing traveler's diarrhea and found that probiotics, including S. boulardii, had the effect of reducing the risk of traveler's diarrhea. 95 patients with traveler's diarrhea in different age groups (19~69 years old) were cured within 5 days after treatment with S. boulardii. Although there are few relevant reports, it is enough to indicate that S. boulardii has a good effect on the prevention and treatment of traveler's diarrhea. Duman et al. evaluated S. boulardii and found that it can reduce the risk of diarrhea in patients receiving triple therapy of Helicobacter pylori (H. pylori). The eradication rate of H. pylori was increased to 80% after the triple therapy is supplemented with S. boulardii. S. boulardii induced morphological changes in H. pylori cells leading to cell damage and reduced H. pylori colonization by 12% in pediatric patients. It can be seen that S. boulardii can effectively reduce H. pylori infection and reduce the side effects of triple therapy.
Table 1 Recommended Clinical Dosage of S. Boulardii
In addition, S. boulardii can be also used in the fermentation of dairy products. Mohamed's study found that S. boulardii had a synergistic effect with pre-selected lactic acid bacteria in the fermentation process of kiwifruit juice fortified milk (4% v/v), which could increase the contents of lactic acid and acetic acid. Meanwhile, the addition of S. boulardii also increased the production of tryptophan and glutamic acid by ≤5.40 mg/L and ≤12.09 mg/L, respectively.
Angel has made some achievements in the research and development of S. boulardii. The S. boulardii Bld-3 strain selected and preserved by Angel has reached 20 billion/g of live bacteria, and its survival ability in the simulated human intestinal environment has been confirmed through the intestinal survival rate - in vitro simulation experiment. Moreover, it can significantly increase the content of short-chain fatty acids in the environment and have a positive regulatory effect on the intestinal flora in the environment. Yao et al. also confirmed the therapeutic effect of S. boulardii Bld-3 on dextran sulfate sodium (DSS)-induced ulcerative colitis (UC) in mice.
S. boulardii has all the advantages of yeast (high quality protein, B vitamins, minerals and dietary fiber, low fat, cholesterol and calories) and has own unique efficacy, making it play a great role in clinical nutrition for animals and humans, and can be also used in dairy product processing. It is believed that with further in-depth study on S. boulardii, its application scope will be wider.
| Published by Gong Shiyu Engineer of Nutrition and Health Division |
About Angel Human Health:
Specialized in yeast and fermentation, AHH is committed to developing innovative, differentiated, science-based functional ingredients and customized solutions, to help our customers get enduring success, as well as contribute to a healthier and sustainable world together.
About Angel:
Angel Yeast Company is a high-tech listed company specializing in yeast and biotech. Product business covers Yeast and Baking, Yeast Extract-Savoury, Nutrition & Health and Biotechnology fields. It is one of the world's leading companies in the yeast industry. Angel has 12 holding subsidiaries and provides products and services for more than 150 countries and regions.
Press Contact:
ANGEL YEAST CO., LTD
Address: 168 Chengdong Avenue, Yichang, Hubei 443003, P. R.China
Tel: +86 717 6369570
Email: Nutritech@angelyeast.com








